Enjoy who you are
Love yourself
Home-use products
Our home-use products bring the best of our advanced and innovative technologies to the comfort of our clients’ homes.
Learn moreLove your clients
Professional products
Our professional products are based on extensive clinical experience, technological innovation and clients’ feedback.
Learn moreGo ahead,
Appreciate your skin
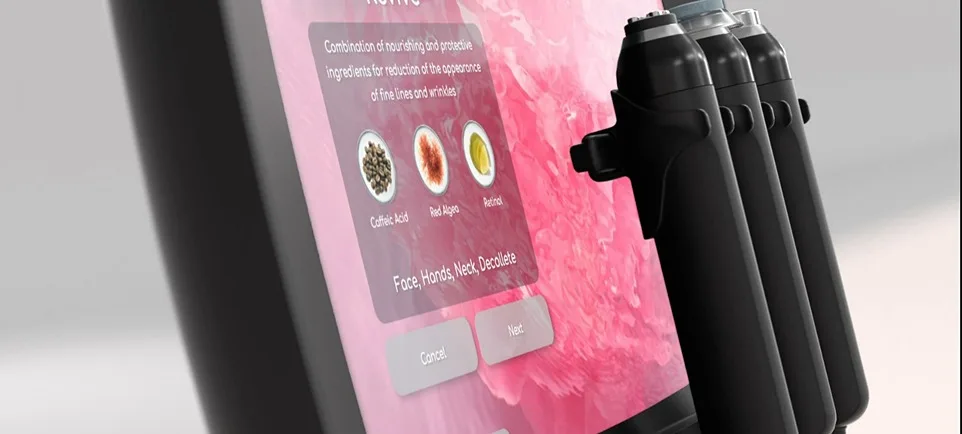
Geneo Oxypods combine ground-breaking concepts in biology, chemistry and technology within one little capsule.
The Intelligence of Your Body
Our products take biology one step forward, encouraging the natural processes of the body to revive, rejuvenate and enhance from within.

PROFESSIONAL
DIVINE PRO

PROFESSIONAL
GENEO X

PROFESSIONAL
MAXIMUS X

HOME
TRIPOLLAR INSPIRE

HOME
TRIPOLLAR PRISM

HOME
TRIPOLLAR STOP VX2 GOLD
Body Wisdom
We constantly challenge ourselves to create the most advanced, innovative and effective medical aesthetic devices that provide the best solutions for our clients
Stay updated
Get in touch
Thank You!
We’ll be in touch soon